

In addition, cells release signals and molecules to adjacent cells, tissues and distant organs, including lipid vesicles and globules containing other molecules (proteins, DNA etc.), and in doing so, they can condition host micro- and macro-environments. Thus, cell membranes are important filters that provide a cellular barrier and continuity, while selectively transmitting signals, nutrients and substances from outside to inside cells and then to various cellular organelles.
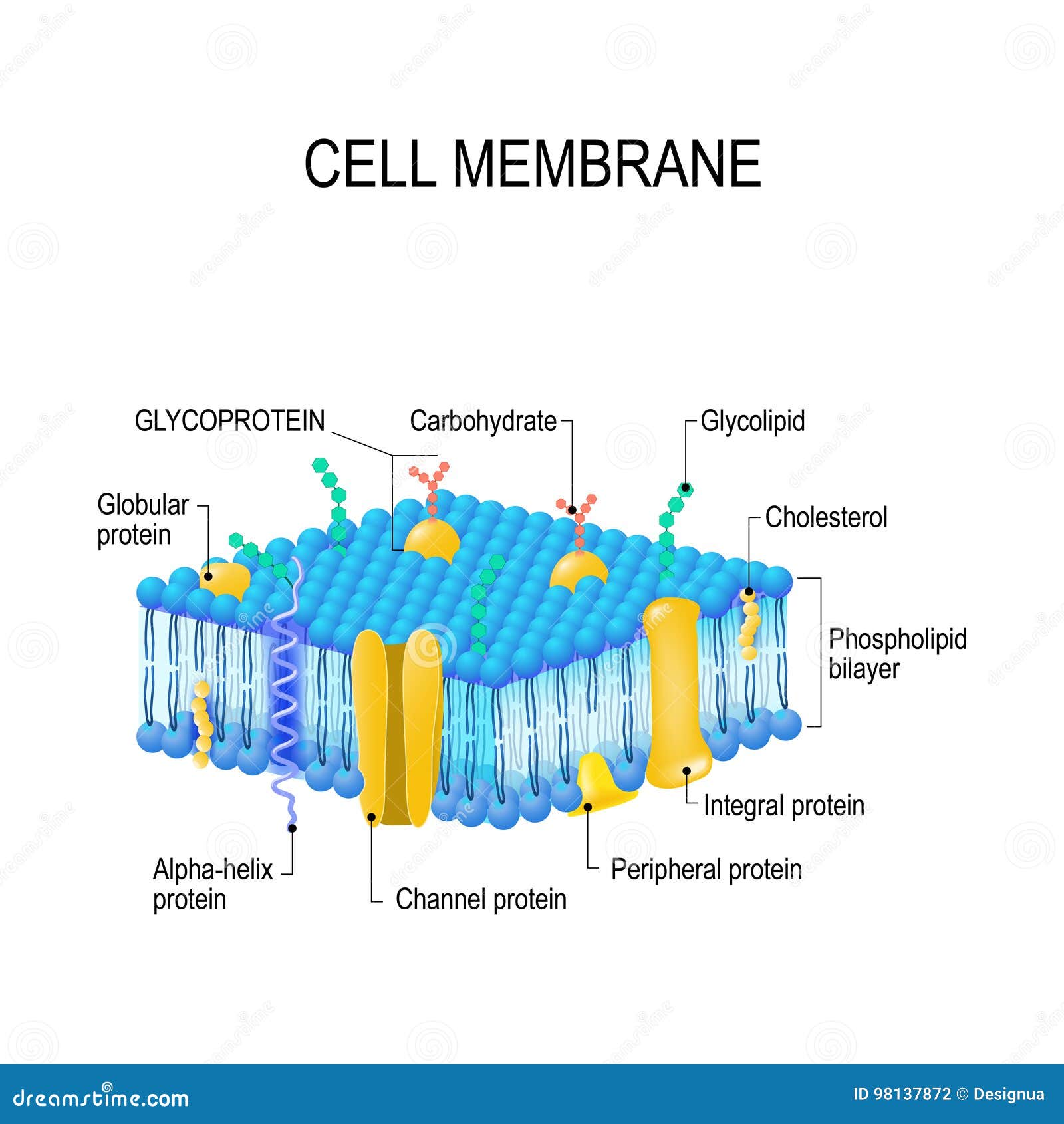
Cell membrane interactions with extracellular molecules determine how individual cells process nutrients, initiate cellular signaling and respond to and maintain normal cellular physiology. In this Special Issue, the use of membrane phospholipids to modify cellular membranes in order to modulate clinically relevant host properties is considered.Ĭell or plasma membranes are the first cellular barriers encountered by extracellular ions, molecules, lipid vesicles and globules, viruses and other cells. They can also be externalized in a reverse process and released as extracellular vesicles and exosomes. Various lipid globules, droplets, vesicles and other membranes can fuse to incorporate new lipids or expel damaged lipids from membranes, or they can be internalized in endosomes that eventually fuse with other internal vesicles and membranes. However, the fluid regions of membranes are very important in lipid transport and exchange. The presence of specialized membrane domains significantly reduced the extent of the fluid lipid matrix, so membranes have become more mosaic with some fluid areas over time. In addition, lipid–lipid and lipid–protein membrane domains, essential for cellular signaling, were proposed and eventually discovered. Subsequently, the structures associated with membranes were considered, including peripheral membrane proteins, and cytoskeletal and extracellular matrix components that restricted lateral mobility. This simplified version of cell membrane structure was never proposed as the ultimate biomembrane description, but it provided a basic nanometer scale framework for membrane organization. Integral membrane proteins can transform into globular structures that are intercalated to various degrees into a heterogeneous lipid bilayer matrix. The Fluid–Mosaic Membrane Model accounted for these and other properties, such as membrane asymmetry, variable lateral mobilities of membrane components and their associations with dynamic complexes. These thermodynamically untenable structures did not allow lipid lateral movements independent of membrane proteins. Early cell membrane models placed most proteins external to lipid bilayers in trimolecular structures or as modular lipoprotein units.


 0 kommentar(er)
0 kommentar(er)
